Background
The past two decades have seen a revolution in the science that underpins new health technologies. Many new technologies offer hope for previously untreatable conditions and potential step changes in the outcomes of care for many others. Regulators committed to supporting the translation of the breakthroughs in biomedical knowledge into the clinic often approve new medicines on the basis of immature evidence, in terms of both the quantity and the quality of evidence for effectiveness and safety.
At the same time, the processes for manufacturing many of these new technologies are considerably more expensive than for conventional therapies such as small molecule pharmaceuticals. This, along with factors driving up research and development costs, means that many of these new technologies arrive at market with prices that are of magnitudes greater than previously encountered.
Improvements in population health status and the effectiveness of healthcare are also leading to increasing demand for treatment, as people live long enough to develop conditions associated with aging. As a result, not only the price but also the budget impact of introducing new technologies is much larger than was historically the case, with some technologies representing a genuine threat to the financial viability of smaller healthcare payers.
For reimbursement authorities charged with making protecting and promoting the health of the population they serve, the tension between the promise of these new technologies and the relative paucity of evidence that the promise will be fulfilled inevitably gives rise to the question ‘What if the resources are consumed but the promise is not fulfilled?’ The risk of making the wrong decision, the decision uncertainty, and how policy makers have responded are the focus of this article. The remainder of this article is constructed as follows. The Decision Uncertainty in Healthcare Resource Allocation section briefly reviews the concept of decision uncertainty and then outlines the factors that contribute to decision uncertainty in the context of value-based healthcare resource allocation decision processes. The Designing Patient Access Schemes section reviews the literature on their design and implementation, proposing a typology for differentiating them according to their objective and the mechanism for managing the decision uncertainty. The Evidence for the Success or Otherwise of Patient Access Schemes section considers the evidence for the success or otherwise of different types of scheme. The Linking Research and Reimbursement Decisions section considers recent developments linking research and reimbursement decisions, whereas Section Value-Based Pricing and Decision Uncertainty considers the degree to which a value-based pricing reimbursement framework might meet the needs of policy makers to manage the decision uncertainty around new and potentially innovative technologies. Eventually, Opportunities and Challenges in Emerging Decision Uncertainty Management Frameworks section considers emerging risk management frameworks from the perspective of health systems, manufacturers, and patients.
Decision Uncertainty In Healthcare Resource Allocation
Decision uncertainty can be thought of as the risk of making the wrong decision, the probability that the observed costs and outcomes will be sufficiently different from the expected costs and outcomes, that the option not chosen would have been better than the one chosen. Given that this is an unknown and one-off event, such probability is inherently a Bayesian rather than a frequentist concept. In the context of healthcare resource allocation decisions, the decision uncertainty is an accumulation of the uncertainty in the characterization of parameters in the decision problem, the characterization of the objectives of the decision process and the decision rules that flow from those objectives.
The extent to which decision makers need to take account of decision uncertainty is largely determined by the expected cost of making the wrong decision. The expected cost of making the wrong decision is determined by the probability of making the wrong decision given the currently available evidence and the expected health gains foregone and additional costs incurred due to making that decision. This is also known as the expected value of information (VoI). Where the expected cost of making the wrong decision is small, it is unlikely that investing in measures to reduce it will be justified, even when the decision uncertainty is high. Similarly, if the health gains foregone and costs incurred if the decision proves wrong are high, but the decision uncertainty is low, then investing in ameliorating mechanisms is unlikely to be efficient. However, with innovative technologies, healthcare systems are often facing a combination of high levels of decision uncertainty combined with high expected cost of uncertainty. In these circumstances, explicit consideration of mechanisms to reduce the expected cost of uncertainty is almost required for good stewardship of limited resources.
The simplest mechanism for reducing the cost of uncertainty is to reduce the cost of the technology; historically this has arguably been the most frequently used strategy. However, there are constraints on when this can be used and the degree of discount that is feasible. In pharmaceuticals, parallel imports, where drugs are bought in a low-price market and then sold in a higher price market, with the intermediary receiving the price premium rather than the manufacturer, are often cited as a reason for not using price discounts to manage risk. In some cases such as biologics, the cost of production of the technologies may mean that the scale of discount required to reduce the cost of uncertainty to an acceptable level may be such that it would threaten its commercial viability.
If the expected cost of uncertainty cannot be reduced to acceptable levels through discounts, then there are a number of alternative drivers of the cost of uncertainty that can be addressed within the reimbursement decision process, some of which relate to the evidence for the technology, some to its clinical application, and some to the decision criteria used. Still others relate to the manner in which the technology is paid for by the health system.
Evidence And The Expected Cost Of Uncertainty
The uncertainty in the evidence base for a new technology is the type of uncertainty that people consider most readily. It is recognized that for most new treatments, the evidence is provided by studies whose participants are very different from the patients who will be treated in everyday clinical practice; the length of follow-up in the studies is typically too short to provide any insight into the long-term effectiveness of the technology, and the number of participants is likely to be too small to uncover any rare but severe safety problems with the treatment. For technologies that treat previously untreatable conditions, it may even be the case that the health system will be substantially uncertain about how many patients have the condition of interest. To some degree the only way to be confident about how valuable a technology is, is to use it in a large number of ‘typical’ patients in routine clinical practice, and to do so for a reasonably long time. However, to do this simplistically entails the health system taking on the expected cost of the uncertainty. The development of Access with Evidence Development schemes, which are discussed in more detail in the section Decision Uncertainty and Innovative Payment Mechanisms, are an attempt to square the circle of generating real-world evidence on the value of a new technology while reducing a health system’s exposure to the expected cost of the uncertainty.
Decision Uncertainty And The Clinical Application Of A New Technology
Frequently the indications for the application of a new technology, as described in the license or the summary of product characteristics, tend to inclusivity. Where the value of the new technology is uncertain, reimbursement authorities will frequently seek patient subgroups within the licensed indication for whom there is evidence of a greater expected benefit than for the whole population. Even though, by definition, the uncertainty in the estimate for the effectiveness of this group is greater because the estimate is based on less data, the expected cost of uncertainty is reduced because the smaller number of patients reduces the budget impact and the expected value for the subgroup is more clearly below the decision threshold.
When reimbursement authorities are not in a position to identify a patient subgroup for whom to approve reimbursement, they may choose to impose a cap on the total budget impact of the technology. This strategy addresses the expected cost of uncertainty first by limiting the total expenditure directly and second by creating an indirect incentive for clinical practice to focus the utilization of the technology on those patients for whom it will be most beneficial. This strategy is particularly attractive where there is a significant risk of off-label use of the technology. In some cases, reimbursement authorities have linked manufacturers’ payments to the achievement of predicted cost offsets in other areas of the budget, a form of financial risk sharing scheme that is distinct from the more widely known effectiveness-based risk sharing schemes. The advantage of this approach is that it creates an incentive for the manufacturers to discourage off-label usage as such use is less likely to generate the targeted cost offsets.
Decision Uncertainty And Reimbursement Decision Criteria
Healthcare resource allocation decisions are rightly subject to challenge by the patients, clinicians, and manufacturers who are affected by them. Arguably the most frequent challenge made to these decisions is that the value of the benefits of the technology has not been adequately captured in the evidence considered. Decision processes that assume the value of health gains are independent of the characteristics of the recipient, and are frequently challenged to take account of special factors such as the (lack of) alternative treatments, the severity of the condition, the imminence of death, the rarity of the condition, the age of the people affected, and even whether the health gain is produced in an innovative manner. All of these special factors attempt to shift the decision threshold and in doing so reduce the probability that the technology will prove not to be of good value, and thereby drive down the expected cost of uncertainty. The evidence base to specify decision criteria is both sparse and of variable quality. What evidence there is does not speak strongly to value premia for many of the proposed factors, but neither do they support a pure health gain maximization strategy. As a result the social legitimacy of these amendments to decision criteria frequently rests on the democratic legitimacy of the decision makers.
Decision Uncertainty And Innovative Payment Mechanisms
A final group of responses to decision uncertainty in reimbursement decision processes has been the development of new payment strategies. Conventionally, healthcare systems have paid for technologies in full prior at the time of their consumption, with the exception of large capital equipment such as magnetic resonance imaging (MRI) machines and surgical robots where leasing arrangements have been deployed. The effect of this is that all the risk associated with the uncertainty of the technology is transferred from the manufacturer to the health system before the outcome of treatment is known. Two distinct types of payment mechanism responses to this problem have been observed; the first operates at the individual patient level, whereas the second operates at the group level. Such schemes attempt to address decision uncertainty by reducing the expected budget impact and reducing the risk that payment will not produce the anticipated results.
Individual-level schemes – often referred to as payment by results, risk sharing, or Patient Access Schemes – link payment to outcomes for the individual patient. Beyond this basic shared characteristic, the specifics of the schemes vary. In some, initial treatment is provided free of charge but only patients who respond to treatment continue with treatment that is paid for. With extremely expensive treatments, such as those for very rare diseases, the monitoring of response to treatment is sometimes a continuous process, so that if a patient stops responding, the funding can be stopped. In other schemes, patient treatments are funded up to a maximum number of administrations, after which the manufacturer provides the technology free of charge, the presumption being that only patients who are responding to treatment will remain on treatment beyond the maximum number of administrations.
Group or population-level schemes tend to be referred to as Access with Evidence Development, Coverage with Evidence Development, or risk-sharing schemes. Under such schemes patients receiving the treatments will provide data on response to therapy as part of the scheme. These data are then used to inform a review of the reimbursement decision at a specified point in time. The review may lead to a change in the price or indeed a change in the reimbursement status. In principle, these group-level schemes offer a limit on the budget impact if the technology does not prove to be as valuable as hoped, and produces additional data to reduce the decision uncertainty.
Designing Patient Access Schemes
The range of policy responses to decision uncertainty in healthcare resource allocation has given rise to a relatively large number of labels in a remarkably short period of time: including risk sharing, coverage with evidence development, access with evidence development, patient access, and only with research (OWR). Behind all of these labels is a shared intention of achieving prompt patient access to the technology under consideration while attempting to ameliorate the expected cost of uncertainty associated with the reimbursement decision. Although the number of Patient Access Schemes is large, and the literature that comments around individual schemes is notable, substantial research on the principals that should inform their design and implementation is scarce.
The National Institute for Health and Clinical Excellence (NICE) has described the principals that will guide their assessment of a Patient Access Scheme, and the Commission for Medicaid and Medicare Services in the US is developing on guidance on the design of Coverage with Evidence Development schemes. In a similar vein, the International Society for Pharmacoeconomics and Outcomes Research is developing good practice guidance on the design of performance-based risk-sharing schemes. A Canadian group produced a consensus statement on the design of access with evidence development schemes in 2010, although this was based on a review of schemes that had worked well or not so well, rather than any clear theoretical framework.
Work on a theoretical framework for Patient Access Schemes is anchored in Decision Science, more specifically the VoI framework. Claxton and colleagues have focused on developing criteria to identify the efficient choice, for decision makers, between open and conditional reimbursement for a technology, and when the choice is for conditional reimbursement, to identify whether reimbursement should be only in research (OIR), i.e., only patients involved in the research have access to the technology, or OWR, which provides access for all patients as long as the research goes forward. Importantly, they have demonstrated that awaiting further research can be the correct decision even when the expected incremental cost effectiveness ratio is below the cost effectiveness threshold. It is the magnitude of the uncertainty, the budgetary impact of reimbursement, the feasibility undertaking the necessary research while the technology is generally available, and the reversibility of the investment that drive the value of further OIR or OWR Patient Access Schemes.
The work of Claxton and colleagues has tended to consider the burden of the uncertainty associated with specific parameters in a decision problem and not the details of the research that would be required to address that uncertainty. Their work is complemented by a series of publications from Willan and colleagues, who have developed methods for establishing the value of clinical trial research, taking due account of the time it takes for the research to report, costs incurred, and the value of any health gain foregone while the research is completed. More recently, Hall and colleagues placed this type of analysis in the decision framework used by Claxton, showing how to assess the expected value of an OIR and OWR Patient Access Scheme from the perspective of the healthcare payer. It is noteworthy that the work of Hall and colleagues indicates that OWR strategies are only likely to be an efficient use of health system resources when the expected cost of uncertainty is relatively low. These developments proffer real benefits to agencies interested in Patient Access Schemes as a means to reduce the expected costs of uncertainty by improving the evidence base for future decisions. However, this is only one component of the cost of uncertainty and only one of a number of possible objectives for a Patient Access Scheme.
Strictly, the VoI framework is not focused on Patient Access Schemes; rather it considers the most efficient means for generating additional evidence to inform a reimbursement decision. Achieving prompt patient access may be the result of such analyses, but it is not the primary concern. The primary concern is the risk that uncertainty in the evidence base may lead to the inappropriate reimbursement of an inefficient technology or the inappropriate rejection of an efficient one. The results of a well-designed and well-implemented VoI study may expedite or delay general patient access to a treatment. However, VoI does provide a framework for designing policy responses to uncertainty in the evidence base.
It may be useful, therefore, to differentiate between policy responses that have the reduction of uncertainty in the evidence base as their primary aim and policy responses whose primary aim is patient access to therapy. Within the former category, there are schemes that allow patient access to therapy while additional evidence is developed and schemes that constrain patient access in order to enable collection of additional evidence. In the latter category there are a range of schemes and they differ according to their secondary objective: (1) Patient Access Schemes that seek to reduce the cost per treated patient – in essence price discount schemes, (2) Patient Access Schemes that seek to limit the budget impact of the technology, (3) schemes that seek to target expenditure on those patients who respond to therapy, and (4) schemes that seek to develop evidence to inform future reimbursement decisions.
If policy responses that focus on the reduction of uncertainty are labeled Type 1, and responses that focus on patient access Type 2, six distinct categories of policy response to uncertainty in the evidence base can be defined: Type 1 OIR, Type 1 OWR, Type 2a, Type 2b, Type 2c, and Type 2d.
Understanding the specific type of scheme may help predict or explain observed policy responses to additional evidence. For example, Type 2d schemes may appear to be equivalent to Type 1 OWR schemes. However, the difference in the primary objective – reduced uncertainty versus patient access – is likely to lead to different policy responses to the same evidence. The UK multiple sclerosis risk sharing scheme is likely a Type 2d scheme. It was explicitly established to enable patient access to therapies that were not considered good value while at the same time collecting further evidence on the effectiveness of the therapies. Thus, accumulated evidence that might support changing the reimbursement status has received a very cautious policy response.
Evidence For The Success Or Otherwise Of Patient Access Schemes
The volume of Patient Access Schemes reported in the literature may well be the best evidence of their success. Decision makers keep returning to the schemes as a means of breaking the deadlock between patients and manufacturers on the one side and the limited resources of the healthcare system on the other. However, evidence that Patient Access Schemes have delivered affordable population health gain, or information to inform subsequent research decisions, is notable by its absence. Very few schemes have published reports of any data that have been collected and even fewer have reported estimates of the population health gain attributable to a scheme. By contrast, there is a substantial literature reporting problems with the process characteristics of Patient Access Schemes.
The Banff Workshop in 2010 identified problems with the process as one of the major impediments to success, where success was defined as observable changes in reimbursement and/or clinical practice in response to the evidence accumulated by the scheme. The same workshop, having reviewed published evidence and heard from experts involved in a number of different health systems, produced a consensus statement that emphasized the importance of governance in the establishment of schemes, if the intended objectives were to be achieved.
Linking Research And Reimbursement Decisions
Once a technology is licensed for use in a healthcare system, the typology of policy responses described in Section Designing Patient Access Schemes provides a useful framework for considering the linkages between further research and reimbursement. However, the reimbursement decision is also the mechanism that a healthcare payer has for signaling their willingness to pay for new technologies, and hence there is, conceptually at least, a link between reimbursement decision making and prelicensing research.
Considering postlicensing research and how it relates to reimbursement, the value of any research is dependent on the willingness of decision makers to change the reimbursement status in response to additional evidence. There is little, if any, convincing evidence that data collected as part of Type 2
Patient Access Schemes lead to changes in reimbursement status. The trial of lung volume reduction surgery, which many consider the first example of a Medicare Patient Access Scheme, produced strong evidence that the intervention was not effective and yet coverage of the procedure was not revoked. Similarly, the UK multiple sclerosis risk sharing scheme changed the rules in response to the first release of data, which indicated that the treatments were not effective, thereby avoiding a review of its reimbursement status.
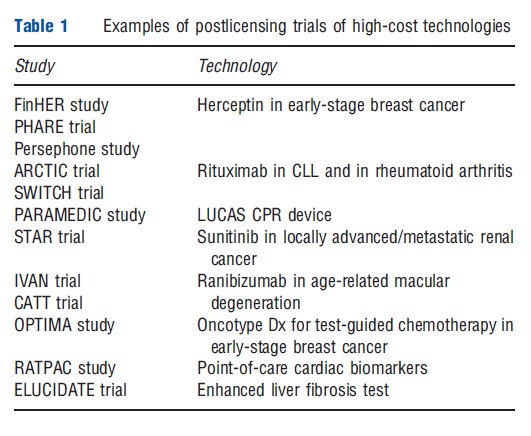
There is an emerging interest in postlicensing trials of extremely expensive technologies such as Herceptin, rituximab, avastin, and lucentis (Table 1). Often these studies are at least partly funded by the healthcare payers and can often be cost saving in their own right, irrespective of the results that they provide, due to the cost of the technologies. However, there remains the challenge for decision makers of changing the reimbursement status when the studies report. In the absence of a commitment from decision makers to act on the evidence generated by such research, there must be questions about the ethics of enrolling patients to trials with a view to benefiting others, if the link to future reimbursement is fractured by the healthcare payers’ reluctance to reverse a previous positive reimbursement decision. The recent decision by the UK NICE to reverse its positive recommendation for denosumab for prostate cancer is an encouraging development in this area.
Although postlicensing responses to uncertainty in the evidence base for new technologies are useful, they are a response to the problem that new technologies arrive at market that is often ill-suited to the needs of reimbursement decision makers. As market access is increasingly dependent on reimbursement rather than licensing and clinical use decisions, commercial considerations should lead companies engaged in developing new healthcare technologies to link their prelicensing research and development activities to the evidence needs of reimbursement decision makers. The VoI framework that Claxton and colleagues have developed relatively fully for quantifying the value of research from the perspective of healthcare systems is increasingly being considered as a mechanism for evaluating prelicensing research investments.
The central observation that additional research, through reducing decision uncertainty, impacts on the probability of a positive or negative reimbursement decision is pertinent to both health systems and technology developers. However, the costs and benefits of positive and negative reimbursement decisions are very different. Specifying the cost and payoff functions for technology developers is arguably more complex than for healthcare systems, but it is likely to be at least as valuable. In a prelicensing context, additional research delays the time to licensing and thus the start of an income stream from the technology. It also ‘burns’ patent life and thus reduces the expected time from licensing to the onset of generic competition. These are costs that need to be captured in the evaluation of the investment. The characterization of benefit is also more complicated as evidence will inform decisions in a portfolio of healthcare systems. Each system is likely to operate different decision criteria and will differ in terms of the revenue stream to be expected conditional on a positive decision, reflecting different epidemiology and pricing policies. Willan and colleagues have started to examine these issues, but it remains a very immature literature. That said, it arguably has the greatest potential for matching research and development investment to technologies that will produce effects that patients and health systems will value and hence pay for.
A specific policy barrier to the wider utilization of these methods in the development of new technologies is the role of licensing authorities such as the Federal Drug Administration in the USA and the European Medicines Agency in Europe. Conventionally these organizations have focused on evidence of safety and efficacy rather than incremental value. Although the licensing authorities retain this focus and their approval continues to be a necessary but not sufficient condition for market access, technology developers will be understandably reluctant to adopt new strategies for designing and prioritizing prelicensing research and development. Although there are increasing communications between licensing and reimbursement authorities, the two communities appear to be still getting acquainted rather than developing coherent and complementary strategies focused on aligning research and the evidence needs of those who must decide whether and how much to pay for new technologies.
Value-Based Pricing And Decision Uncertainty
The concept of value-based pricing for health technologies is a relatively new one, but interest in it has grown rapidly. Initially, value-based pricing was thought of as a change in the mechanism for establishing the price of a treatment. Many healthcare systems are price takers. Reimbursement authorities consider whether a technology is of good value using the price that the manufacturer stipulates. The idea of value-based pricing is a simple one – why don’t reimbursement authorities consider a technology and then specify the price at which it would represent good value. However, as consideration of how to operationalize this concept for real-world decisions has gathered pace, the debate about how to assess the value of a technology has intensified. In large part these discussions have covered the same arguments as the literature regarding the adequacy of the quality-adjusted life-year, as a measure of the effect of a technology, for use in cost effectiveness analysis. Equity arguments for value premia reflecting inter alia severity of ill-health, rarity, availability of alternative therapies, extensions of life at the end of life, and cause of disease have all been proposed as components of the assessment of value. The use of formal multicriterion decision-making processes has been proposed as a mechanism for capturing these disparate components of value. Although there is uncertainty if not outright ignorance about the relative and absolute value weights for these components of value, a multicriteria approach to resource allocation decisions is not a policy response to uncertainty.
Value-based pricing can be considered a policy response to uncertainty in that it allows decision makers to identify the price at which the expected cost of uncertainty does not support delaying reimbursement while further research takes place, or limiting reimbursed patient access during the research. However, in the context of the high cost and high levels of uncertainty associated with biologic, metabolomic, and genomic technologies, it is possible, even likely, that the price at which these conditions are met could have significant implications for the sustainability of private investment in health technology development and even the production of the technologies. In response to this concern, health policy makers are being encouraged to consider a premium for innovation and to allow prices to be revised upward if data from use of the technology in practice either reduce the uncertainty relating to its expected value or indicate that its actual value is greater than previously thought. The latter would represent a significant departure from current practice, where the price charged for a technology at launch is the highest price point, and subsequent developments will at best maintain the price and likely lead to price reductions. The data capture infrastructure required for the routine application of price adjustments based on observed effectiveness would be substantial, and it would be interesting to see whether there would be a symmetrical reluctance to increase price in the face of reduced uncertainty, reluctance to reduce price when evidence has suggested a technology has been less valuable than claimed. The incentives for gaming the system when the evidence of value is not derived from well-conducted randomized controlled trials will likely be significant, especially for therapies for common disorders.
The innovation premium is the added value attributed to a technology that does something that current technologies do not do, over and above the value attached to its effectiveness compared to currently available technologies. The justification for such a premium would likely rest in either the option value of subsequent alternative applications of the technology to meet other currently unmet needs or, when there is no evidence to support such an expectation, the value of the hope for such application. What is clear is that the innovation premium is a reward for ‘newness’ and thus likely to be highly and positively correlated with uncertainty. As such, the innovation premium works in the opposite direction to the expected cost of uncertainty and increases the likelihood of a positive reimbursement decision at any given level of decision uncertainty. In the context of population health promotion, the magnitude of the innovation premium should depend on the option value of future potential applications. However, when healthcare organizations have implicit or explicit industrial policy objectives, such as the National Health Service in the UK and the Commission for Medicare and Medicaid Service in the USA, the magnitude of premium may partially reflect these considerations also.
A further potentially problematic characteristic of value-based pricing as a mechanism for addressing uncertainty is the creation of different prices for the same technology in different markets. Manufacturers are understandably concerned about differential pricing creating opportunities for parallel imports of their technologies acting as a downward pressure on their global revenue. Consumers in high-price environments such as the US, Germany, or France might procure their therapies in lower price markets such as Canada, Poland, and Spain. However, there are additional challenges associated with value-based pricing schemes for manufacturers. First, because knowledge is essentially a public good, health systems that are not engaged in a value-based pricing schemes may be able to free ride, even if the specifics of the additional evidence generated is kept confidential, as any change in price will be informative. Second, because healthcare systems differ in terms of budgets and the epidemiology of disease in the populations served, there will likely be much larger variation in prices between health systems than is currently observed, with an associated increase in the uncertainty in the expected return for investors.
Although value-based pricing is intuitively appealing as a response to uncertainty that will not require the reengineering of existing research and development processes, its operationalization for highly uncertain high-cost technologies may not be consistent with the sustainability of research and development investment. Further, its implementation may introduce new parameters into the decision problem about which there is substantial uncertainty from the healthcare payer perspective. At the same time, it may generate additional uncertainty regarding revenue flows for the manufacturers and investors, as prices arrived at through value-based pricing processes may differ markedly between healthcare markets even when the value criteria used are shared. New evidence generated through a value-based pricing mechanism in one system will likely influence prices in other systems; this may not be symmetrical; i.e., health systems may be more likely to ‘free ride’ on knowledge that supports a price cut than knowledge that supports a price rise. It may be that manufacturers would be more attracted to lower but more certain returns on their investment, compared to opening this Pandora’s box.
Opportunities And Challenges In Emerging Decision Uncertainty Management Frameworks
Broadly there are four strategies for addressing the decision uncertainty facing reimbursement authorities driven by the mismatch between the evidence produced by conventional health technology development processes and the evidence required to inform efficient and equitable use of limited healthcare budgets: (1) Patient Access Schemes, which focus on achieving patient access alongside one or more secondary objectives such as per patient cost containment, total cost containment, and targeted use or evidence development; (2) research, which has the reduction of decision uncertainty as its primary objective. This may require that the technology is not available to patients except as part of the research (OIR) or may allow access to the treatment if that does not confound the required research study or is required for the research to proceed (OWR); (3) value-based pricing, which sets the price of the technology at a level that reduces the expected cost of uncertainty associated with reimbursement below the cost of requiring further research; and (4) reengineering the prelicensing research and development process to meet the needs of reimbursement decision makers.
The first three strategies treat the focus of current research and development processes on the requirements of licensing authorities as immutable. They are more or less focused on the needs of the identified patients who will benefit from the newly licensed technology (1 and 3), or the needs of the unidentified patients who will bear the opportunity cost of reimbursing the new technology (2 and 3). The fourth strategy perhaps naively assumes that the structures within which technologies are developed can be redesigned and considers how it might be redesigned to match technology development investments to the objectives of healthcare systems.
The time it takes for investments in research and development to pay off means that policies that address problems with the conventional evidence development processes will be required for many years to come. However, this does not mean that work on developing a more efficient research and development process, focused on developing high-value technologies with ‘reimbursable’ evidence dossiers at the time of licensing, is not worth investment. That said, there are significant challenges to be addressed in developing the VoI framework to inform the design of research and investment processes.
VoI is predicated on a clearly specified payoff function – whether it be population health benefit or revenue from sales. For investors in mid to late-stage clinical trials, the payoff function of interest is conditional on the objectives of the portfolio of healthcare systems that are the clients for the technologies they are seeking to develop. The methods for representing and combining these functions in assessing the value of alternative investments may not be intellectually trivial challenges.
Skeptics are likely to argue that much of the information that is pertinent to the decision problem cannot be known with any confidence so far in advance of the decision, and therefore early-stage VoI analyses are likely to involve as much guess work as knowledge. Although this is true, to use it as the basis for rejecting changes in the approach for designing research and development processes is to assume that a similar degree of guess work is not implicitly or even explicitly involved in the current processes. Given the high failure rate in the research and development process, and the problem with licensed technologies struggling to achieve reimbursement, it seems likely that the current process is based on at least an equally flawed assessment of the values and needs of future healthcare systems.
There are short-, medium-, and long-term challenges facing healthcare systems seeking to take a systematic approach to managing the uncertainty in reimbursement decisions. In the short term, Patient Access Schemes are likely to be more not less prevalent and thus the total value of resources invested is likely to increase. Experience to date does not provide confidence that these schemes are automatically of good value to the health systems that enter into them. Careful design and governance may reduce the cost of uncertainty associated with these schemes. In the medium term, all these schemes rely to a substantial degree on capturing reliable evidence on the impact of therapies on patients in the typical clinical setting. Few health systems currently have routine data capture infrastructure fit for this purpose. The capacity for establishing successful Patient Access Schemes may well be among the more valuable, if less noticed, returns on investing in such infrastructure. On a longer term, all reimbursement authorities in healthcare – innovators, investors, regulators, clinicians, patients, and health systems – need to find mechanisms to align the research and development processes with the needs of all patients, signaling societies’ willingness and ability to pay for health gain, so that the current incentives to invest large sums in high-risk candidates that may produce only marginal health gains are removed, leading to fewer marginal value and highly uncertain technologies being launched.
Bibliography:
- Chen, M. H. and Willan, A. R. (2013). Determining optimal sample sizes for multistage adaptive randomized clinical trials from an industry perspective using value of information methods. Clinical Trials 10(1), 54–62.
- Claxton, K. (2007). OFT,VBP:QED? Health Economics 16, 545–558.
- Claxton, K., Palmer, S., Longworth, L., et al. (2012). Informing a decision framework for when NICE should recommend the use of health technologies only in the context of an appropriately designed programme of evidence development. Health Technology Assessment 16(46), 1–323.
- Conti, S. and Claxton, K. (2009). Dimensions of design space: A decision theoretic approach to optimal research portfolio design. Medical Decision Making 29, 643–660.
- Department of Health (2011). A new value-based approach to the pricing of branded medicines: Government response to consultation. London. Available at: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/ prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_128404.pdf (accessed 12.04.13).
- Desser, A. S., Gyrd-Hansen, D., Olsen, J. A., Grepperud, S. and Kristiansen, I. S. (2010). Societal views on orphan drugs: Cross sectional survey of Norwegians aged 40 to 67. British Medical Journal 341, c4715.
- Eckermann, S., Karnon, J. and Willan, A. R. (2010). The value of information: Best informing research design and prioritization using current methods. PharmacoEconomics 28(9), 699–709.
- Eckermann, S. and Willan, A. R. (2008). The option value of delay in health technology assessment. Medical Decision Making 28, 300–305.
- Eckermann, S. and Willan, A. R. (2009). Globally optimal trial design for local decision making. Health Economics 18, 203–216.
- Griffin, S., Claxton, K. and Welton, N. (2010). Exploring the research decision space: The expected value of information for sequential research designs. Medical Decision Making 30, 155–162.
- Grossman, G. M. and Lai, E. L. (2008). Parallel imports and price controls. RAND Journal of Economics 39(2), 378–402.
- Hall, P. S., Edlin, R., Kharroubi, S., Gregory, W. and McCabe, C. (2012). Expected net present value of sample information from burden to investment. Medical Decision Making 32(3), E11–E21.
- Linley, W. G. and Hughes, D. A. (2012). Societal views on nice, cancer drugs fund and value-based pricing criteria for prioritising medicines: A cross-sectional survey of 4118 adults in Great Britain. Health Economics, doi:10.1002/hec.2872.
- McCabe, C., Claxton, K. and O’Hagan, A. (2008). Why licensing authorities need to consider the net value of new drugs – addressing the tension between licensing and reimbursement. International Journal of Technology Assessment in Health Care 24, 140–145.
- McCabe, C. J., Stafinski, T., Edlin, R., Menon, D. and Banff AED Summit (2010). Access with evidence development schemes: A framework for description and evaluation. PharmacoEconomics 28(2), 143–152.
- McKenna, C. and Claxton, K. (2011). Addressing adoption and research design decisions simultaneously: The role of value of sample information analysis. Medical Decision Making 31, 853–865.
- Menon, D., McCabe, C. J., Stafinski, T., Edlin, R. and Signatories to the Consensus Statement (2010). Principles of design of access with evidence development approaches: A consensus statement from the Banff Summit. PharmacoEconomics 28(2), 109–111.
- Mohr, P. E. and Tunis, S. R. (2010). Access with evidence development: The US experience. PharmacoEconomics 2, 153–162.
- NICE (2008). Guide to the methods of health technology appraisal, 3rd ed. London: NICE.
- Sculpher, M. J., Claxton, K., Drummond, M. and McCabe, C. (2006). Whither trial-based economic evaluation for health care decision making? Health Economics 15(7), 677–687.
- Stafinski, T., McCabe, C. and Menon, D. (2010). Funding the unfundable: Mechanisms for managing uncertainty in decisions on the introduction of new and innovative technologies into healthcare systems. PharmacoEconomics 28(2), 118–142.
- Towse, A. (2010). Value based pricing, research and development, and patient access schemes. Will the United Kingdom get it right or wrong? British Journal of Clinical Pharmacology 70(3), 360–366.
- Towse, A. and Garrison, L. P. (2010). Can’t get no satisfaction? Will pay for performance help? Toward an economic framework for understanding performance-based risk-sharing agreements for innovative medical products. Pharmaco-Economics 28(2), 93–102.
- Wailoo, A., Tsuchiya, A. and McCabe, C. (2009). Why weighting must wait: Incorporating equity concerns into cost-effectiveness analysis may take longer than expected. Pharmaco-Economics 27(12), 983–989.