Advertising Of Prescription Drugs
Between 1980 and 2009, expenditures on prescription (Rx) drugs in the US increased from $12 billion to $250 billion, representing an increase of 1974% (see Figure 3).
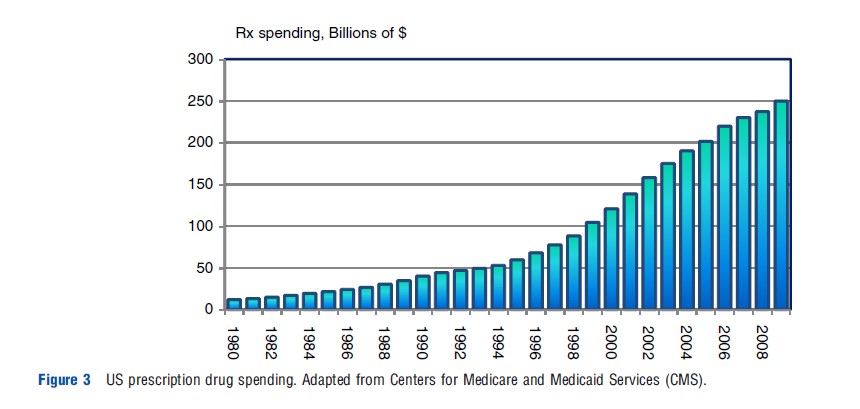
Most of the increase until the mid-1990s followed the growth in national health expenditures (NHE). However, since around 1995 spending on Rx drugs has outpaced the growth in NHE, making it one of the fastest growing components of health care costs. Consequently, the share of drug spending in NHE roughly doubled between 1994 and 2004, from 5% to 10% (Centers for Medicare and Medicaid Services CMS; see Figure 4).
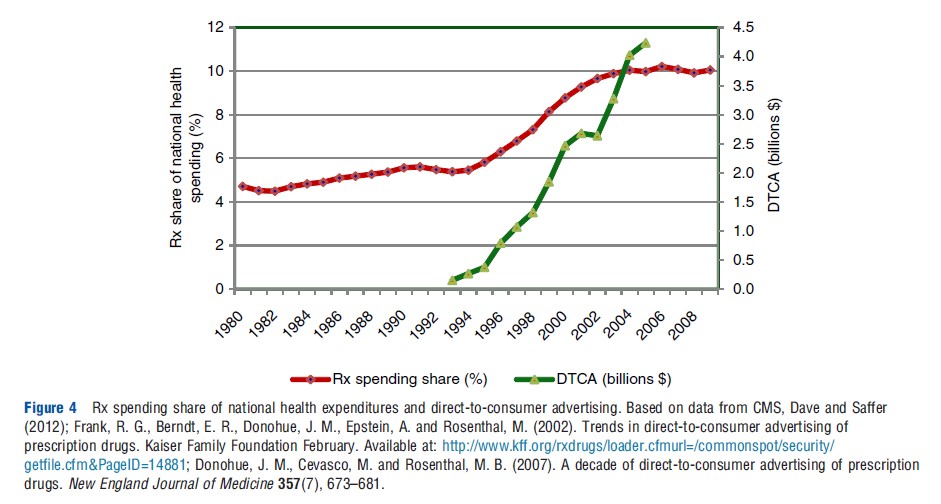
The growth in the share of prescription drug expenditures has coincided with the growth in pharmaceutical promotion, which increased from $11.4 billion in 1996 to $29.9 billion in 2005 (Donohue et al., 2007). The promotion-to-sales ratio for the pharmaceutical industry is approximately 20%; this compares to an all-industry average of 4–5%. Pharmaceutical products tend to have experience attributes, a low price elasticity of demand (because of the presence of insurance and third-party payers), and a relatively high sales-advertising elasticity – all of which contribute to a high advertising and promotion intensity.
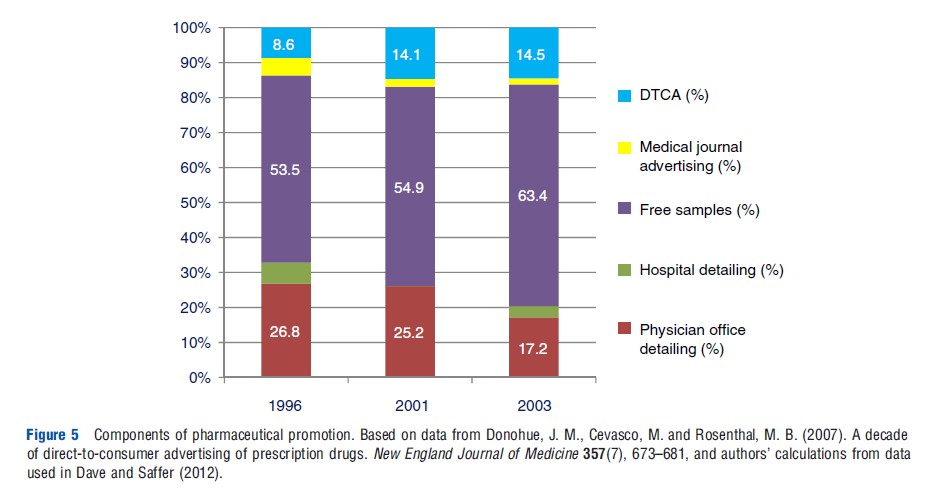
Promotion of prescription drugs is generally limited to patented drugs. It includes direct-to-consumer advertising (DTCA) on broadcast and print media as well as direct-to-physician promotion (DTPP) through visits by company representatives to physician offices (known as detailing), free samples provided to physicians and advertising in professional journals. Although DTPP still comprises most of the promotional budget, the largest relative increase in promotion between 1995 and 2005 resulted from the expansion of DTCA into broadcast media. The share of total promotional spending allocated to DTCA increased from less than 1% in the early 1990s to 8.6% in 1996 to 14.5% in 2003 (see Figure 5).
This expansion of DTCA was precipitated by the FDA’s clarification of the rules governing broadcast advertising in 1997 and 1999, making it feasible for companies to promote via television and radio advertisements. For a number of years, the FDA had guidelines requiring the advertiser to provide detailed information on usage and risks that is contained in the drug’s FDA-approved product label insert, thereby confining ads to print form. The new regulations now require broadcast advertisements to include only ‘major statements’ of the risks and benefits of the drug along with directions to alternate information sources for full disclosure. This clarification of what constitutes adequate disclosure removed a major barrier that had initially made TV and radio advertisements infeasible.
Specifically there was no broadcast advertising in 1993, but it now comprises the primary form of DTCA – amounting to $2.55 billion in 2005.
These new regulations remain a controversial policy and are facing increased scrutiny from Congress and consumer groups. Currently only the US and New Zealand permit broadcast DTCA. At the heart of this debate is whether pharmaceutical promotion and advertising are welfare-promoting. The pharmaceutical industry claims that such advertising educates patients on potential treatment options, opens up lines of communication between the patient and the physician, and can even increase patient–physician contact or expand appropriate treatment for under treated conditions, consistent with the informative view of advertising. Congressional leaders have contended that DTCA raises prescription drug costs, consistent with brand differentiation and the persuasive view of advertising, and requested that the policy be revisited. Some consumer groups maintain that consumers may be harmed by misleading advertising and that the recent expansions in DTCA are responsible for the increases in expenditures on prescription drugs.
Growth in prescription drug spending is broadly driven by increases in utilization and price, and shifts in the composition of drugs being used, all of which may be impacted by DTCA. A comprehensive assessment regarding the welfare effects of pharmaceutical advertising and promotion requires information on three broad but related issues: (1) effects on primary versus selective demand; (2) effects on price; and (3) effects on competition. To inform on the first question, many prior studies gave focus on how DTCA and DTPP have affected pharmaceutical sales and patient adherence. Rosenthal et al. (2003) studied brands in five therapeutic classes using aggregated US monthly time-series data from August 1996 to December 1999. They employed an instrumental variables methodology to account for the endogeneity of DTCA and concluded that consumer advertising was primarily effective in raising sales for the entire therapeutic class. Other studies have also noted this market-expansion effect of DTCA, and suggested that DTCA may be more effective in increasing aggregate class demand than in increasing the demand for a particular drug (Iizuka and Jin, 2005, 2007).
These studies combine broadcast and non-broadcast DTCA into a single aggregate measure, and utilize older data from a time-period when DTCA was just starting to take off and much of it still comprised non-broadcast forms. This may obscure certain effects since the shift in FDA guidelines specifically applied only to broadcast DTCA; the composition of DTCA has increasingly shifted away from print and toward television and radio advertising as broadcast DTCA became more feasible as a form of promotion for the pharmaceutical industry. Second, both of these forms of DTCA may be expected to have differential effects on pharmaceutical prices and sales.
Dave and Saffer (2012) utilized monthly data on all prescription drugs in four major therapeutic classes from 1994 to 2005, thereby exploiting the period enveloping the FDA’s shift in regulations as a natural experiment and exogenous shock to consumer advertising. They separately analyzed the effects of broadcast and non-broadcast DTCA. Based on drug fixed effects models, they found that broadcast DTCA did impact own-sales with an elasticity of 0.10, and this response is higher relative to non-broadcast DTCA. This study also found some evidence that class-level DTCA may raise sales for the non-advertised drugs. Assuming that physicians are prescribing an equally effective drug, this may be a spillover benefit of DTCA in some cases because non-advertised drugs tend to be older and also cost less.
Directly bypassing the potential endogeneity of advertising, Kravitz et al. (2005) examined how DTCA impacts the prescribing behavior of antidepressants in a randomized control trial setting. Standardized patients, mostly professional actors, were assigned to visit physicians and make a specific brand request (referring to a DTC ad), a general drug request, or no request. Results pointed to the role of brand-specific DTCA in raising own-demand by leading to a prescription for that brand, as well as in raising overall class demand.
Additional evidence on the demand effects of DTCA is also provided by studies that examine patient adherence. For instance, Bradford et al. (2006), using patient-level data from 1998 to 2004 merged with DTCA information at the national and market levels, found that higher levels of DTC television advertising of statin treatment was significantly associated with improvements in the likelihood of attaining cholesterol management goals for at least some patients. Donohue et al. (2004) studied claims data for depressed patients between 1997 and 2000 matched with information on DTCA. They found that consumer advertising of antidepressants was associated with an increase in the number of people diagnosed with depression who initiated medication therapy and a small increase in the number of individuals treated with antidepressants who received the appropriate duration of therapy.
Studies have also examined the impact of advertising aimed at health-care providers, which historically has been the primary form of promotion used by the pharmaceutical industry. Berndt et al. (1995), for instance, considered the role of detailing, medical journal advertisements and DTCA in the market for antiulcer drugs before the shift in FDA guidelines. The DTCA examined in this study is very limited and confined only to print media because the study predated the FDA’s shift in regulations that made broadcast DTCA feasible. They found the strongest demand effect for detailing and the smallest effect for DTCA. Many other studies also confirmed larger effects of physician-directed promotion relative to those for consumer-directed promotion.
Overall, most of these studies point to positive demand effects of DTCA and DTPP, and generally find that DTCA has stronger class-level effects whereas DTPP has stronger brandspecific effects. There is some suggestive evidence from studies utilizing newer data that DTCA may also have some brandspecific effects, particularly broadcast DTCA, though all studies point to DTPP being more effective relative to DTCA in raising sales. Some of the research also highlights a potential benefit of DTCA – that is, encouraging consumers to seek treatment and take their medications as prescribed.
With respect to the effects of advertising and promotion on price, the evidence is more limited. This paucity of research partly derives from the difficulty in obtaining salient measures of Rx drug prices because of the presence of third-party payers and unobserved rebates from drug manufacturers to third-party payers.
As underscored by the discussion on the three views of advertising, the potential effects on price primarily depend on the strength of scale economies in production and on the impact of advertising on the price elasticity of demand. Under the persuasive view of advertising where the shift in demand becomes relatively more inelastic, advertising raises price as long as there are no strong economies of scale in production to counteract the inelastic demand. Under the informative view of advertising, prices are predicted to decrease because demand would become relatively more elastic.
The few studies that have focused on advertising-induced price effects appear to be in accord with the persuasive view. Rizzo (1999), for instance, found that increased detailing efforts among antihypertensive drugs reduced the price elasticity. This reduction may consequently result in higher prices, though Rizzo did not examine the direct link between detailing and price. The study was based on pooled annual data from 1988 to 1993, which predates the DTCA policy shift, and only considers promotion to physicians. Law et al. (2009) examined pharmacy data for Plavix (an antiplatelet drug used to prevent stroke and heart attack in at-risk patients) from 27
Medicaid programs over the period 1999–2005. Plavix initiated DTCA in 2001. This study found that, although there was no change in the preexisting trend in demand, there was a sustained increase in cost per unit of $0.40 (11.8%) after the expansion in DTCA.
Dave and Saffer (2012), utilizing a larger sample of all Rx drugs in four therapeutic classes, also found that DTCA raised the average wholesale price, though the estimated elasticity was of a relatively small magnitude (0.04). Consistent with the positive impact on price, this study also found that the consumer price response became relatively more inelastic during the period when DTCA was expanding. Saffer and Dave presented simulations suggesting that expansions in broadcast DTCA over 1994–2005 accounted for 19% of the overall growth in prescription drug spending, with two-thirds of this impact driven by an increase in demand and the remainder because of higher advertising-induced prices.
One challenge faced by these empirical studies concerns the simultaneity between advertising and pricing decisions. For instance, Bhattacharya and Vogt (2003) presented a model of joint price and promotion determination over the drug’s life cycle. The dynamic profit maximizing strategy for the firm was to initially employ a relatively high level of promotion and to set a relatively low price. These levels would not only increase current quantity demanded, but also raise future demand because high promotion and low prices increased the physicians’ and the consumers’ stock of knowledge about the drug. In subsequent periods, promotion could be decreased to lower costs and price could be raised to increase revenue.
This trajectory of higher prices and lower advertising over the drug’s life cycle is also consistent with the Dorfman-Steiner (1954) condition for optimal advertising discussed in Section ‘Overview’; the optimal advertising-to-sales ratio is a positive function of the elasticity of sales with respect to advertising and is inversely related to the elasticity of sales with respect to price. Thus, the decline in advertising over the drug’s life cycle is consistent with an age-related decline in the sales-advertising elasticity (Berndt, 2006). It is also consistent with an increase in the price elasticity as the drug ages and newer drugs enter the therapeutic class. A positive association between advertising and price inelasticity may thus reflect causality in both directions – for persuasive goods, advertising may make demand more inelastic, but ceteris paribus more inelastic demand also leads to a higher optimal level of advertising.
While both Rizzo (1999) and Dave and Saffer (2012) attempted to address this simultaneity through additional controls, the results should be interpreted in the context of the limitations noted. Nevertheless, these studies point to certain anticompetitive effects of Rx drug promotion. Further evidence is gleaned from studies that have investigated the effects of advertising on entry in the pharmaceutical markets. Scott Morton (2000) found that advertising by branded drugs before patent expiration and generic entry may have a very small deterrence effect on subsequent generic entry depending on the type of advertising, though this effect becomes insignificant in models which instrument for advertising. In a classic study, Benham (1972) found that eyeglass prices were substantially higher in states that prohibited all advertising relative to states with no restrictions. Prices were slightly higher in states that allowed only non-price advertising than in states with no restrictions. This strand of the literature suggests that non price advertising by the Rx industry may exert some small upward pressure on prices and possibly have anticompetitive effects, though the evidence is far from conclusive and requires further study.
In summary, DTCA has emerged as a marketing force in the US healthcare system and is only expected to grow along with expenditures on prescription drugs. Although the debate surrounding DTCA is unlikely to be resolved anytime soon, DTCA should be evaluated both in terms of its costs as well as its benefits. The benefits derive from improved health because of increases in the number of individuals using prescription drugs and increased adherence with drug therapy. Detecting and treating health conditions at an earlier stage, through primary care, may also be more cost-effective relative to treatment at a later stage through acute care. Pointing to another potential benefit of promotion, Kwong and Norton (2007) found that detailing (but not other types of advertising) may have a significant positive effect on the number of new products entering into clinical development, with markets for chronic disease with high levels of detailing being more attractive to pharmaceutical firms.
Studies that show advertising-induced market expansion effects generally interpret these findings as welfare-improving. Although there was certainly an element of improved adherence and expanded treatment underlying the market expansion, David et al. (2010) showed that increased levels of promotion and advertising lead to increased reporting of adverse medical events for certain conditions. This suggests that promotion-driven market expansion could raise the risk that the drug is prescribed inappropriately. In addition to potential misuse, the costs of DTCA also result from increased drug prices and increased use of more expensive drugs in place of equally effective lower-priced drugs. Higher drug and health care expenditures in turn can raise insurance premiums and may lead to a larger prevalence of uninsured.